
Dr. Amy Sarjeant received her BS in Chemistry from the College of William and Mary in 1995 while studying the magnetic properties of metal hydrates. In 1999 she received her Ph.D. in Chemistry from Northwestern University, studying solid state compounds of uranium and thorium chalcogenides. Dr. Sarjeant has worked in the pharmaceutical industry, worked for instrument manufacturers, and has most recently served as Research Assistant Professor of X-ray crystallography at Northwestern University in Evanston IL. Amy has been deeply involved with the crystallographic community in North America, co-hosting the American Crystallographic Association (ACA) small molecule summer school, serving on the US National Committee for Crystallography, and has been elected to the ACA Council as Vice President in 2016.
In July 2015, Amy joined the CCDC as Outreach and Education Manager with a focus on fostering collaborations with academic researchers and educators. She has broad experience in small molecule X-ray crystallography having determined over 350 structures since her Ph.D. Most recently she has focused on the structure determination of highly challenging Metal—Organic Framework materials. Amy also has a deep passion for crystallography education and is committed to furthering this discipline throughout undergraduate and graduate curricula.

The Cambridge Crystallographic Data Centre (CCDC) is the home of small molecule crystallography data and is a leader in software for pharmaceutical discovery, materials development, research and education.
The CCDC compiles and distributes the Cambridge Structural Database (CSD), the world's repository of experimentally determined organic and metal-organic crystal structures. We also produce associated knowledge-based application software for the global community of structural chemists, delivered through the CSD-System, CSD-Discovery, CSD-Materials and CSD-Enterprise.
Originating in the Department of Chemistry at the University of Cambridge, the CCDC is now a fully independent institution constituted as a non-profit company and a registered charity. With over 50 years of scientific expertise the CCDC has a strong track record in basic research through more than 750 peer-reviewed publications.
The CCDC employs around 70 staff in Cambridge, UK and Piscataway, New Jersey, USA and hosts occasional visiting scientists and students who are working on projects related to the Cambridge Structural Database or other CCDC products.
Open any chemistry textbook for any course and you’re likely to see quite a few. Read an article about a new pharmaceutical candidate, and one will pop up again. Watch a show about almost any scientific discipline and high resolution images will abound. “Of what?” you are asking. Crystal structures, of course! As scientists who deal in molecular structure, we are used to seeing 3D images of pharmacophores and proteins, of OLEDs and MOFs. These visual representations are our stock and trade. We care about them for a variety of reasons: compound identification, shape recognition, structure-property relationships. We rely on crystallography as a technique to help us get our jobs done, but we can also use the information described by crystal structures as a tool to teach chemistry.
The Cambridge Structural Database (CSD) comprises over 800,000 small molecule crystal structures. Curated and maintained by the Cambridge Crystallographic Data Centre (CCDC), the CSD has become the go-to resource not only for small molecule chemists but for those studying macromolecular science as well. In addition to providing 3D images of each structure, the CSD contains a wealth of statistical information about coordination environments, bond distributions, and intermolecular interactions. By choosing our examples well, we can illustrate a variety of chemical principles by turning to the information contained in the CSD.
Researchers and educators at Newcastle University have produced a series of worksheets that make use of the CSD’s Teaching Subset (freely available through the Mercury software platform and the online Access Structures portal). For example, a worksheet on VSEPR theory leads students through the difficult concepts of using electron count to predict coordination geometry. Corresponding lab modules challenge students to draw out the structures of various molecules and ions based on their CSD refcodes. Another tutorial explains the concepts of aromaticity, with worksheets directing students to use the CSD to measure and compare bond lengths of various aromatic and non-aromatic compounds. There are many more theory and worksheets available, which explain everything from isomerism to chelation effects – all of which can be demonstrated with examples from the CSD.
These worksheets have already been well received by students and teachers alike. From a high school Chemistry teacher in the UK, “I do think that it is fantastic that not only has a good resource been made but also the worksheets help give teachers exemplars of how they can use them in their lessons.”

A 3D model of a fullerene encapsulating a Tungsten complex. Courtesy of Christine Beavers, Advanced Light Source, Lawrence Berkeley Labs.

This 3D model of a perovskite structure was generated by loading the coordinates from the single crystal structure determination into Mercury.

This 3D-printed MOF structure shows the highly porous nature of such materials.
This 3D-printed MOF structure shows the highly porous nature of such materials.
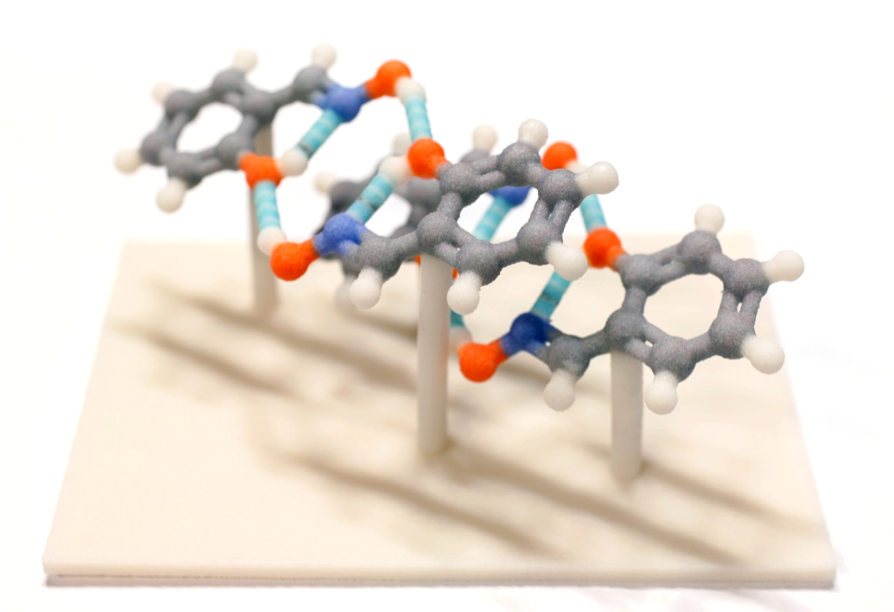
We all remember those plastic model kits from our general chemistry courses. Filled with tetrahedral carbons, octahedral connectors, red and blue spheres for oxygen and nitrogen, we used these to demonstrate all manner of chemical principles. Yet, while these idealized geometries show the theoretical arrangements of atoms in molecules, they fail to show how molecules can really behave and how complex they can become. Students often find it easier to grasp a new idea if they can physically grasp a model. What better way to demonstrate chemistry and the intricacies of molecular structure than with tactile models of real-world compounds? Now that 3D printing has become an affordable resource for most college campuses, and even individual dabblers, it is possible to print copies of molecules for use in the classroom.
With the help of CCDC’s Mercury program, you can now produce a 3D printer file from any structural representation you can visualize on-screen. Any set of coordinates imported into Mercury – either from the 800,000 structures in the CSD or coordinates of your own molecules – can be turned into a hand-held model. Now it is easy to show the deformation that occurs when aromatic rings coalesce into Buckyballs, the steric hindrance that makes a particular catalyst work, the pi-stacking interactions that occur in OLED compounds, and the large porous cavities that have brought MOFs to the forefront of chemistry and engineering. All of these phenomena are difficult to comprehend from the flat pages of a text book, and even from a rotating image on a computer screen. These complex structures are difficult or impossible to build from model kits, yet with a 3D printed model, students are able to see and feel these structures, voids, and interactions on a human scale.
The CCDC is currently exploring methods to print a variety of other interesting compounds. We have envisioned the use of highly flexible materials to produce stretchable and bendable molecules which can help explain the vibrational modes of spectroscopy to young students. We are investigating the production of mechanically interlocked molecules (MIMs) such as catenanes and rotaxanes, to illustrate the switchable and rotatable properties of these compounds.
The possibilities for learning experiences from 3D models are as vast as the educators willing to use them. We are always interested in hearing stories of those who have used 3D models for educational purposes and happy to collaborate to produce more.
In the coming months the CCDC will be producing an array of teaching documents to complement core chemical concepts, working with teachers from around the world to shed the light of structural chemistry on such topics. We welcome comments from anyone interested in using such documents, or any of you who have prepared your own, to reach out and collaborate with us. We can be reached at education@ccdc.cam.ac.uk.